May 21, 2019 | Jola Glotzer
Cells surrounding the cancer promote its spread
Two CBC Awardees, Ernst Lengyel and Raymond Moellering, UChicago, contribute to recent research published in Nature about the role of stroma in facilitating metastasis of ovarian cancer cells
In a recent paper published in Nature, titled “Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts,” the authors look into the role played by non-cancerous cells surrounding the ovarian cancer mass in the dissemination of the cancer cells throughout the body. This surrounding tissue, or stroma, appears to have a striking proteomic profile when displaying metastasis-promoting characteristics. Moreover, an enzyme called nicotinamide N-methyltransferase (NNMT) becomes overexpressed in the stroma adjacent to metastatic cancer cells. NNMT can actually be considered a biomarker because its high expression correlates with the poor prognosis. Thus, further study of stroma’s role in promoting cancer spread is needed as this tissue has the potential to become a novel target for cancer therapeutics.
Ernst Lengyel, a CBC awardee, is senior author on the paper, and Raymond Moellering, another CBC awardee, is one of the coauthors. Lengyel received three CBC awards: a Postdoctoral Research Award (2014; renewal, 2015), an Exploratory Workshop – The CBC Ovarian Cancer Workshop (2013) and an HTS Award (2013). Moellering received a CBC Catalyst Award (2017) and was a featured speaker at the 15th Annual CBC Symposium (2017). In addition, he served on the CBC Accelerator Review Board (2018-present). The CBC thanks both scientists for their service and wishes them all the best in their continued endeavors.
Changes in the metabolism of normal cells promotes the metastasis of ovarian cancer cells
UChicago Medicine, At the Forefront | by John Easton, | May 9, 2019
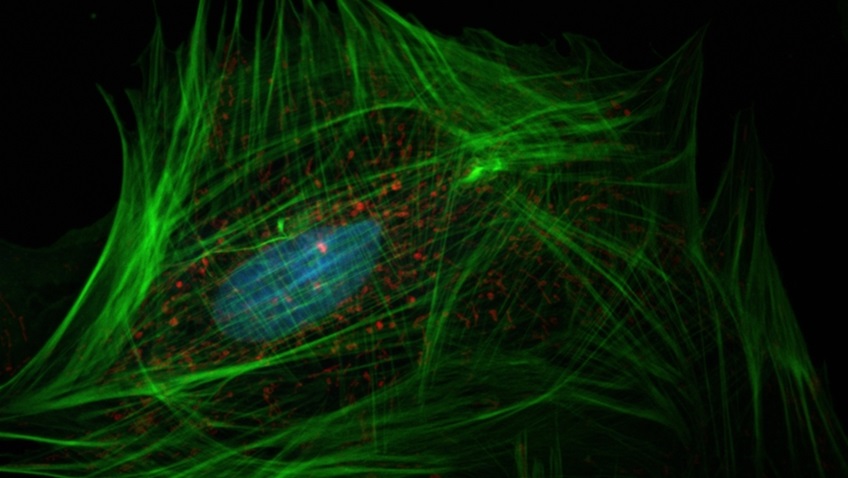
Microscopic image of a fibroblast (top) showing the nucleus (blue), mitochondria (red), and actin cytoskeleton (green). Source: NIH – National Human Genome Research Institute.
A systematic examination of the tumor and the tissue surrounding it — particularly normal cells in that tissue, called fibroblasts — has revealed a new treatment target that could potentially prevent the rapid dissemination and poor prognosis associated with high-grade serous carcinoma (HGSC), a tumor type that primarily originates in the fallopian tubes or ovaries and spreads throughout the abdominal cavity.
High-grade serous carcinoma is the most common form of ovarian cancer and the most deadly. Most patients are diagnosed at an advanced stage when the disease has already spread. Five-year survival is around 50 percent.
“Until quite recently, scientists have focused on the tumor itself,” said the study’s senior author Ernst Lengyel, MD, PhD, professor and chair of obstetrics and gynecology at the University of Chicago Medicine. “Everyone does.”
But given the lack of progress with that approach and the fact the tumors are complex organs comprised of different tumor-supporting cell types (stroma), “we thought it might be better to focus less on the cancer and more on the stroma, the supporting tissue that surrounds the cancer and enables its growth.”
Bigger than the cancer itself
In the May 1, 2019 issue of Nature, the researchers show that the stroma has a major impact on cancer cells. “In this case,” Lengyel said, “it makes them more malignant, more aggressive and more invasive. The stroma is often bigger than the cancer itself.”
In close collaboration with Fabian Coscia, PhD, and Matthias Mann, PhD, from the Max Planck Institute of Biochemistry in Munich and University of Copenhagen, the researchers profiled the expression of more than 5,000 proteins in both normal and cancerous tissues derived from minute amounts of patient biobank material.
“For the first time, we were able to distinguish the molecular changes in the cancer cells from the ones happening in the adjacent stroma throughout disease progression,” explained Coscia. “When we then got our data, we were fascinated to find that the metastatic stroma was characterized by a highly conserved protein signature, as opposed to the cancer cells.”
These metastatic changes were seen in all analyzed patients. The researchers are now trying to understand their functional role during metastasis with the goal of finding novel therapeutic targets.
In the process, they discovered a metabolic enzyme, nicotinamide N-methyltransferase (NNMT), that was highly expressed in the stroma surrounding metastatic cancer cells. They found that NNMT causes widespread gene expression changes in the tumor stroma, converting normal fibroblasts to cancer-associated fibroblasts that support and accelerate tumor growth. Stromal NNMT expression encouraged ovarian cancer progression and metastasis. It was associated with very poor patient outcomes.
‘We put it all together’
The researchers are now using high-throughput screening to find novel ways to inhibit this enzyme.
“One method looked promising,” said Mark Eckert, MD, research assistant professor in obstetrics and gynecology at the University of Chicago and first author of the study. “We have a backbone for several candidate inhibitors. We know our target, we know the structure, we know how to apply this and we have a sense of the direction. We are starting to understand how a normal fibroblast is converted into a cancer-associated fibroblast by this metabolic enzyme.”
They also found that inhibition of NNMT activity reduced or even reversed many of the tumor-promoting effects of cancer-associated fibroblasts. This suggests, they note, that the stroma should be explored as a new treatment target.
Coscia, co-first author on the manuscript and leader of the proteomics analysis, added that “this method may be used to discover other proteins that are important for metastasis and to identify early changes during disease development.”
“When we put it all together,” Lengyel said, “it gave us exciting results. We have linked high-end technology, including proteomics and metabolomics, to improve our understanding of the stroma.”
Citation:
Eckert MA, Coscia F, Chryplewicz A, Chang JW, Hernandez KM, Pan S, Tienda SM, Nahotko DA, Li G, Blaženović I, Lastra RR, Curtis M, Yamada SD, Perets R, McGregor SM, Andrade J, Fiehn O, Moellering RE, Mann M, Lengyel E. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature. 2019 May 1. [Epub ahead of print] (PubMed)
Source:
Adapted (with modifications) from UChicago Medicine, At the Forefront by John Easton, published on May 9, 2019.
Featured CBC Community member(s):
Ernst Lengyel, UChicago
- CBC Postdoctoral Research Award (2014; renewal, 2015):
▸ Differentiating between benign and malignant ovarian tumors using targeted contrast agents for magnetic resonance imaging
PIs: Yilin Zhang (postdoc) and Ernst Lengyel, UChicago - CBC Exploratory Workshop (2013):
▸ The CBC Ovarian Cancer Workshop
PI: Ernst Lengyel, UChicago — Workshop Organizer and Speaker - CBC HTS Award (2013):
▸ HTS Using an Organotypic Culture of Ovarian Cancer Metastasis
PIs: Hilary Kenny and Ernst Lengyel, UChicago
Raymond Moellering, UChicago
- CBC Accelerator Review Board (2018-present):
Raymond Moellering (UChicago) — Board Member - 15th Annual CBC Symposium (2017):
▸ Small Molecule Discovery
Raymond Moellering (UChicago) — Symposium Speaker - CBC Catalyst Award (2017):
PIs: Raymond Moellering (UChicago) and Sarki Abdulkadir (NU) for the project:
▸ Defining Prostate Tumor Malignancy with Activity-Dependent Pet Probes
Previously PUBLISHED ARTICLES ABOUT THE FEATURED CBC COMMUNITY MEMBER(S):
May 4, 2019
▸ Consider slimming down!
CBC affiliate Ernst Lengyel, UChicago, comments in U.S.News on the rise of obesity-related cancer
February 19, 2019
▸ CT45, or cancer/testis antigen 45
Past CBC Awardee, Ernst Lengyel, UChicago, contributes to the discovery of a biomarker that can help predict response to therapy in patient suffering from ovarian cancer
September 28, 2018
▸ Glycogen fuels ovarian cancer spread
Three CBC awardees, Ernst Lengyel, Hilary Kenny and Yilin Zhang, UChicago, co-author new publication in Cell Metabolism
October 6, 2017
▸ Chicago-area holds the fourth highest number of this year’s NIH Director’s New Innovator Awards nation-wide
Three of the four Chicago-area scientists awarded the 2017 NIH Director’s New Innovator Award, Jingyi Fei, Jaehyuk Choi and Raymond Moellering are CBC community members
February 18, 2015
▸ Modeling a 3D Human Ovarian Cancer in vitro to Screen for Metastasis Inhibitors

